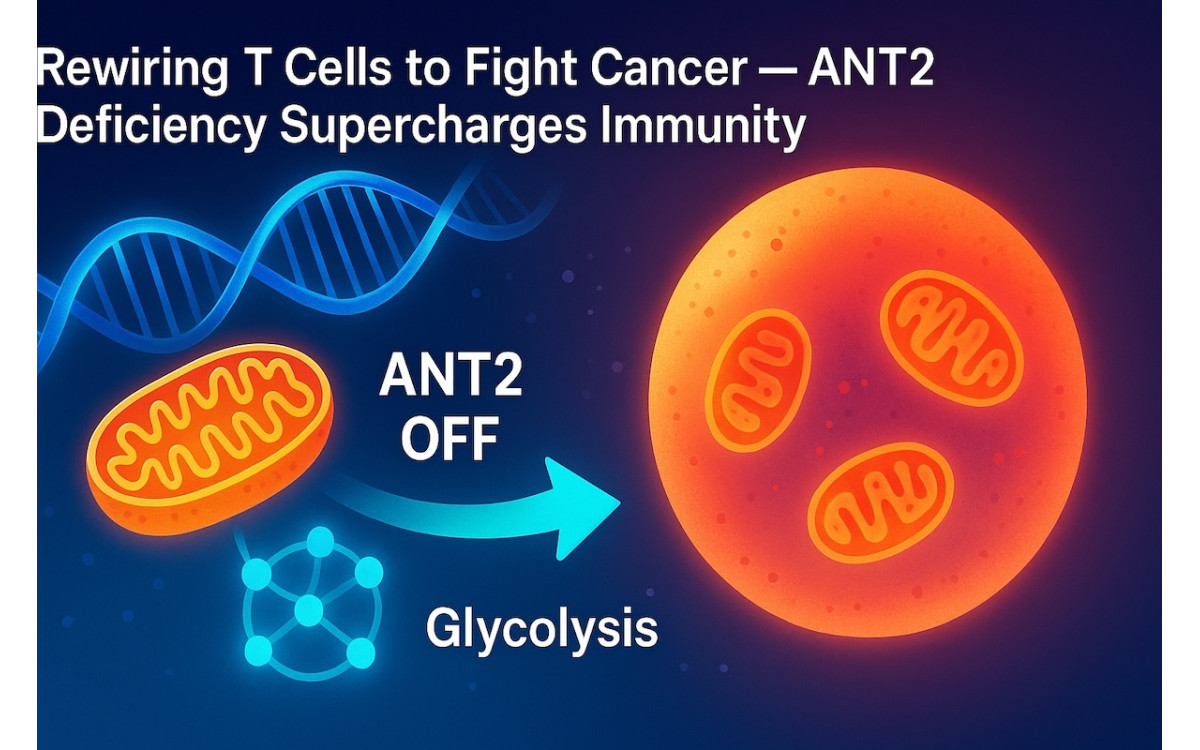
A recent study shows that disabling the mitochondrial ADP/ATP carrier ANT2 shifts T cells into a high-performance metabolic state, enhancing activation, cytokine release, and tumor control in mice. Here’s what it means for next-gen immunotherapy.
Why ANT2?
ANT2 normally exchanges ADP/ATP across the mitochondrial membrane. Blocking ANT2 limits OXPHOS-linked ATP export and forces a beneficial reprogramming toward glycolysis and biosynthetic routes.
What Changes?
- Stronger activation & proliferation
- Higher IFN-γ and effector function
- Greater mitochondrial content / spare capacity
Why It Matters
Metabolically “pre-conditioned” T cells can resist tumor stress, improving adoptive cell therapy and potentially CAR-T outcomes.
Applications in the Lab
- Ex vivo T-cell activation & expansion protocols
- Testing ANT inhibitors to mimic the phenotype
- Optimizing double-digest cloning for vector edits (see Restriction Enzymes)
Translational Outlook
Targeting ANT2 or transiently modulating ANT activity could “tune” T cells before infusion, boosting persistence and function in hostile tumor microenvironments.
Always evaluate safety/off-targets; human translation requires rigorous preclinical validation.